In the world of pH measurement, accuracy is paramount. The right pH analyser can make all the difference in various applications. Dr. Emily Carter, a leading expert in analytical chemistry, states, "Choosing the right pH analyser is critical for reliable data." This highlights the importance of selecting a suitable tool for effective monitoring.
Today, a range of pH analyser tools are available, each designed for specific needs. Some devices offer advanced features but can become overly complex. Others may lack precision, leading to questionable results. It's essential to find a balance between complexity and ease of use. Every detail counts in chemical analysis.
Moreover, it's worth noting that not all tools are built the same. As technology evolves, so do the capabilities of these analysers. Users must remain critical of their choices and continually reassess their tools. Relying solely on past experiences may not suffice in today's fast-paced environment. Therefore, exploring the latest innovations in pH analysers is key to achieving consistent and accurate results.

pH measurement is crucial across various industries. It impacts product quality, safety, and compliance. For instance, in the food and beverage sector, maintaining the right pH level ensures the safety and flavor of products. A study by the Food Safety Authority indicates that 85% of foodborne illnesses are linked to improper pH control. Ensuring pH balance can prevent spoilage and extend shelf life.
In water treatment, pH levels play a vital role in the chemical processes involved. According to the Environmental Protection Agency, water with extreme pH levels can harm aquatic life and affect drinking water quality. Monitoring pH in real-time can enhance treatment efficiency. Moreover, in agricultural practices, soil pH impacts nutrient availability. Research shows that crops may yield 15% more when soil pH is optimally maintained.
Despite the importance of pH measurement, many industries still struggle with inaccuracies. Approximately 30% of pH readings can be erroneous due to equipment limitations or user error. This highlights the need to invest in advanced pH analyzers and training. Continuous monitoring and regular calibration are essential for achieving reliable results. Industries must reflect on their practices and equipment to ensure accurate pH management.
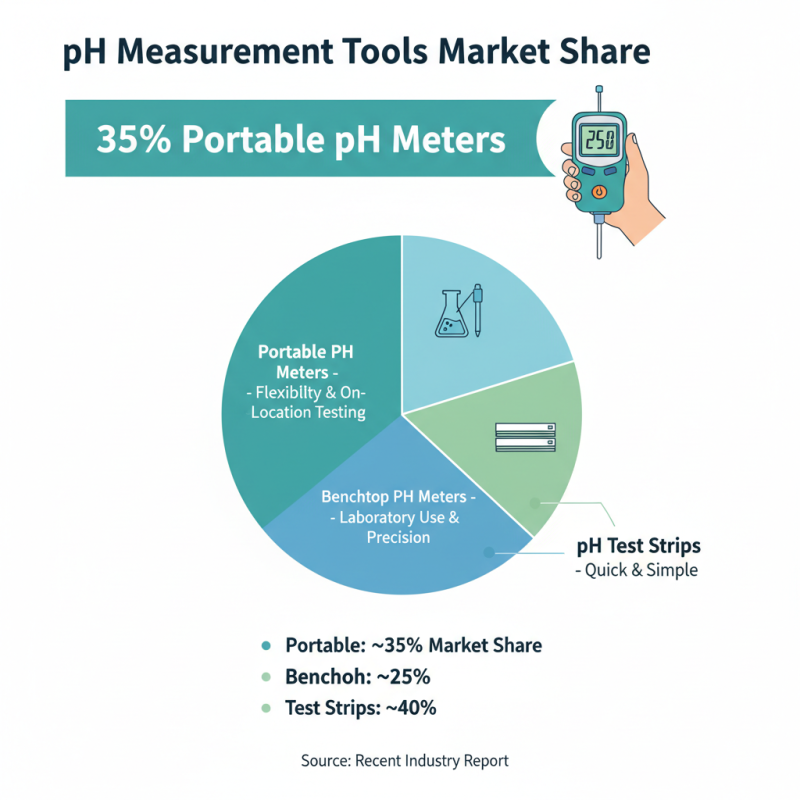
When it comes to pH measurement and monitoring, a variety of tools are available in the market. Broadly, these can be categorized into portable pH meters, benchtop pH meters, and pH test strips. Each type serves specific purposes. Portable pH meters are highly valued for their flexibility. They enable users to test samples in different environments easily. According to a recent industry report, these meters account for approximately 35% of the pH measurement market due to their convenience.
Benchtop pH meters, on the other hand, are suited for laboratory settings. They provide more precise readings and often feature advanced data logging capabilities. The precision of benchtop models can lead to more reliable results. However, they may not always be user-friendly, especially for operators unfamiliar with complex interfaces. In fact, a study indicated that nearly 25% of lab technicians found difficulty interpreting readings from sophisticated benchtop meters.
pH test strips are another widely used option. They are simple and inexpensive, making them accessible for many users. However, accuracy can be an issue. The strips may provide only approximate pH values, which is a problem for critical applications. Research shows that these strips represent around 30% of the market, highlighting their popularity despite some limitations in precision. Users must reflect on their specific needs when selecting the appropriate tool for pH measurement.
When selecting a pH analyser tool, consider specific features for accuracy. An essential feature is a reliable calibration system. Regular calibration ensures consistent results. Look for tools that offer automatic calibration. This saves time and minimizes human error.
Another critical aspect is the display readability. Users need clear, easily interpretable data. Features such as backlit screens improve visibility, especially in low-light conditions. User-friendly interfaces enhance the overall experience. If the tool is complicated, users may struggle.
Finally, consider portability. Instruments should be lightweight and compact. This is particularly important for fieldwork. However, don’t neglect durability. Tools must withstand tough conditions. Yet, some users may overlook this, affecting long-term performance. It's vital to reflect on these features when making a choice.
When selecting pH analyser tools, the variety available can be overwhelming. Different brands and models offer unique features that cater to specific needs. Accuracy, ease of use, and durability are key factors to consider. Some tools provide advanced calibration options, while others emphasize portability for fieldwork. It’s crucial to assess what fits your specific situation best.
Tips for pH measurement: Always calibrate your pH meter regularly. A well-calibrated meter ensures reliable readings. Consider the environment too; temperature can impact measurements. For outdoor use, a waterproof design is essential. Familiarize yourself with the tool’s instruction manual. Sometimes, small details in use can significantly affect results.
Models vary from simple handheld devices to complex lab-grade equipment. Not all tools are user-friendly. Some require a steep learning curve. It’s essential to choose a model that you feel comfortable using. Always check reviews for insights on usability and performance. This can save you from making a costly mistakes.
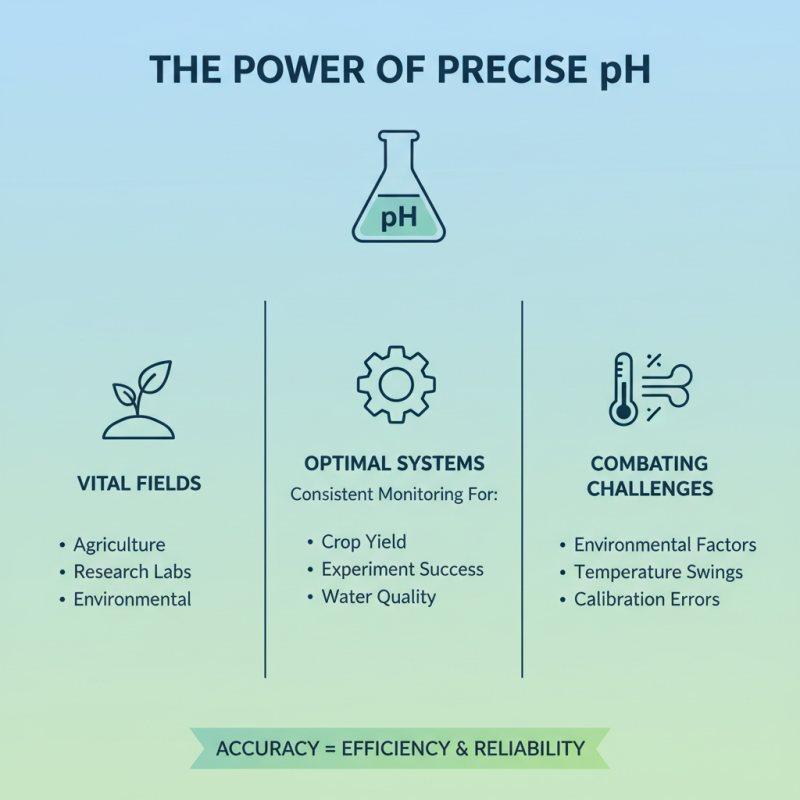
Accurate pH measurement is vital in many fields, from agriculture to laboratory research. Consistent monitoring ensures that systems operate within optimal ranges. However, achieving precision can be challenging. Environmental factors, for example, can affect measurements significantly.
When using pH analyzers, calibration is key. Regularly calibrate your device using standard solutions. Even slight deviations can lead to significant errors. Consider the temperature of your samples too. pH readings can fluctuate with temperature changes.
Tips: Always check electrode condition. A worn electrode can produce unreliable readings. Rinse the electrode with distilled water between uses to avoid contamination. Keep your workspace clean; debris can interfere with measurements.
Data recording is essential for tracking trends. Document measurements diligently. Analyze patterns over time to catch discrepancies early. Establishing a routine can sometimes lead to complacency, impacting accuracy. Regularly question your methods for improvement.